May 20, 2022, Biotime announced that its SARS-CoV-2 Antigen Rapid Qualitative Test has received approval from the China's National Medical Products Administration (NMPA). The details are as follows:
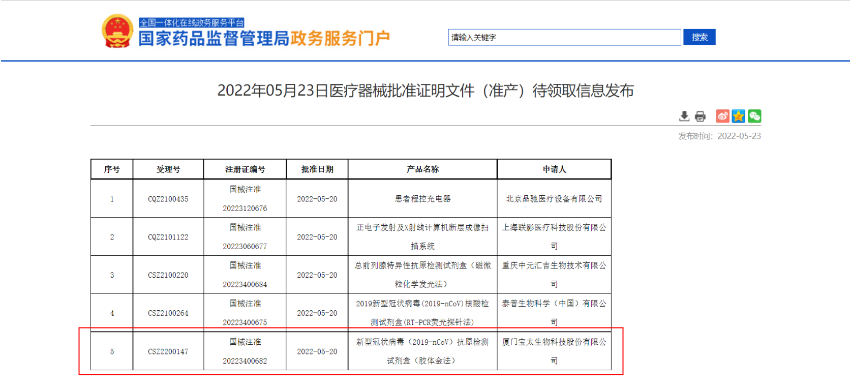
The SARS-CoV-2 Antigen Rapid Qualitative Test has also obtained CE mark for self-testing, which is another effective tool for the company to actively support global anti-epidemic. It can be operated by individuals who collect the front nasal swab sample by themselves, and the result can be quickly delivered in 20-30 minutes.
Since the outbreak of COVID-19, Biotime as a global innovation-driven healthcare company has fully integrated its business and global resources with timely response and actively undertaking the social responsibilities in combating COVID-19 pandemic control and minimizing its impact. Biotime has developed a growing number of diagnostic solutions that help to diagnose and detect the infection, and we will continue to play our role and increase production to support availability of tests globally.