Boronate affinity chromatography (BAC) is a
unique means for selective separation and enrichment of cis-diol-containing
compounds. Cis-diol-containing biomolecules are an important class of
compounds, including glycoproteins, glycopeptides, ribonucleosides, ribonucleotides,
saccharides, and catecholamines. These biomolecules play essential roles in
many life-related processes. Because cis-diol-containing biomolecules are
important target molecules in current research frontiers such as proteomics,
metabolomics, and glycomics, BAC and boronate affinity materials have gained
rapid development and found increasing applications in recent decades.
BAC is a unique mode of affinity
chromatography, in which a boronic acid is used as the ligand for the selective
isolation and enrichment of cis diol-containing compounds. The retention
mechanism mainly relies on the pH-controlled reversible covalent interactions
between cis-diol groups and the boronic acid ligand. As compared to other
affinity chromatographic techniques, BAC exhibits several significant features,
including broad-spectrum selectivity, reversible covalent binding,
pH-controlled binding/release, and fast association/desorption kinetics. Owing
to these merits, BAC is of great value in a variety of fields such as affinity
separation, proteomic analysis, and metabolomics analysis.
HISTORICAL DEVELOPMENT
The history of BAC can be simply divided
into three different periods: early development period before 1970,
approach-forming period 1970–2005, and new development period since 2006.
PRINCIPLE AND BINDING PH
BAC principle relies on the reversible
covalent reaction between cis-diol-containing compounds and boronic acid
ligands. Figure 1 shows a general formula for the interaction between boronic
acid and a cis diol-containing compound. When the surrounding pH is greater
than the pKa value of the boronic acid, hydrolysis of the boronic acid occurs,
resulting in a hybridization status change from trigonal coplanar shape to
tetragonal boronate anion (from sp2 to sp3). The obtained tetragonal boronate
anion can react with cis-diols and form five or six-membered cyclic esters.
When the pH of the surrounding solution is switched to acidic, the boronic
acid-cis-diol complex dissociates, because the binding strength between boronic
acids in trigonal form and cis diol-containing compounds is very weak. Owing to
the pH-controlled reversible covalent reaction, elution of captured analytes in
BAC is very simple, just needing an acidic solution as the eluting buffer.
Alternatively, the release of the captured analytes by the boronic acid ligands
can be realized through adding excessive amounts of competing for
cis-diol-containing molecules such as sorbitol into the loading buffer.
BORONATE AFFINITY CHROMATOGRAPHY
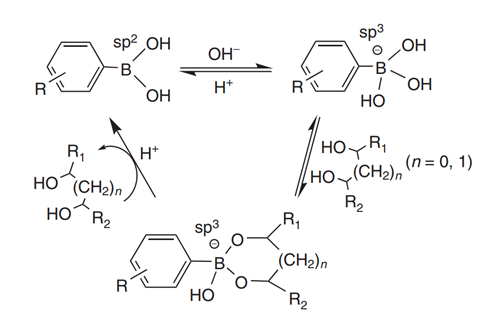
Figure 1 Schematic diagram showing the interaction between boronic acids and
cis-diol-containing compounds.
INTERACTION MECHANISM AND SELECTIVITY
MANIPULATION
Selectivity is an essential concern in BAC.
It is relatively easy to obtain good selectivity for small cis-diol-containing
molecules. However, it is often a challenging task for macromolecules,
particularly glycoproteins. To reach a pure BAC separation, a sound
understanding of the interaction mechanism is indispensable. In addition to
boronate affinity interaction, four secondary interactions, including
hydrophobic, ionic, hydrogen bonding, and coordination interactions, can occur
in BAC. Under certain conditions, secondary interactions may result in
significantly undesirable secondary retention. For example, unprotonated amines
and carboxyl groups can serve as electron donors and thus can coordinate with
boronic acids, which may reduce selectivity. A set of strategies for
selectivity manipulation in BAC. These
strategies can be classified into two categories: choosing or designing
appropriate stationary phases and choosing appropriate binding buffer
composition. The strategies for manipulating the selectivity and related
information are illustrated in Figure 2.
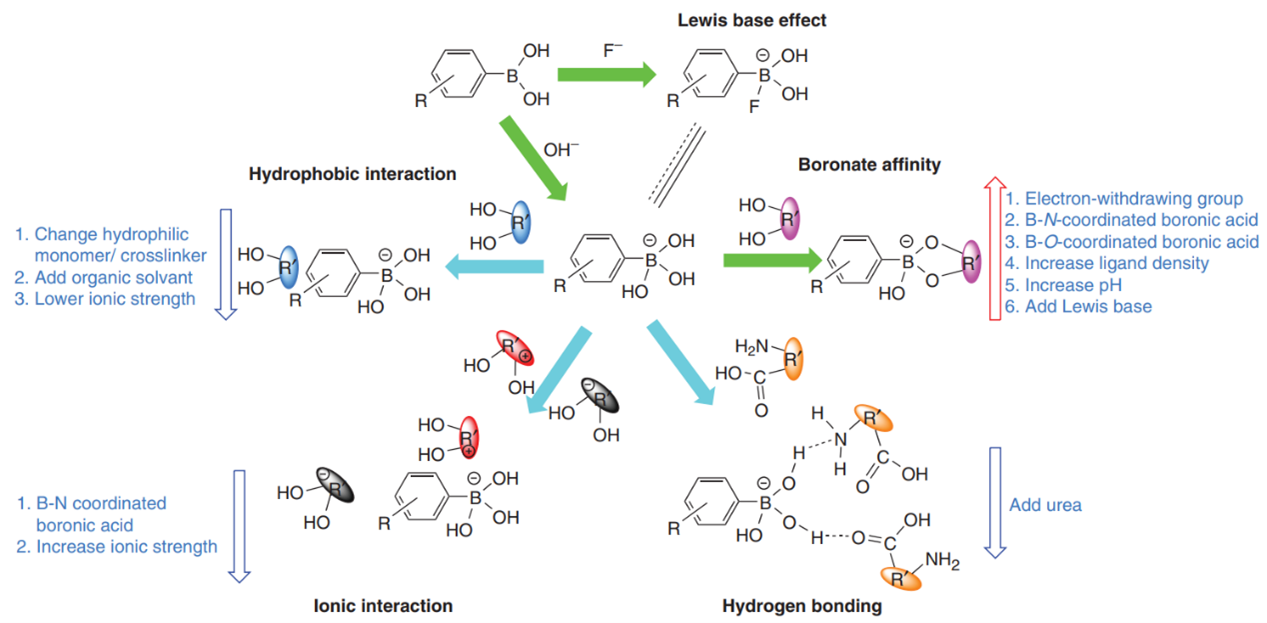
Figure 2 Selectivity manipulation and factor-affecting performance of BAC. Green arrows
mean favorable interaction while cyan arrows mean unfavorable interactions. A
red-up arrow means that the interaction can be enhanced by the factors
specified, while blue arrows mean that the interactions can be suppressed by
the specified factors.
APPLICATIONS
Although BAC appeared as early as 1970, BAC
had not found wide applications until recently. The most important application
before 2006 was the selective isolation of glycated hemoglobin for the clinical
diagnosis of diabetes mellitus. Several fundamental issues, including
selectivity, binding pH, and binding affinity, have been well solved with the rapid
and deep development of BAC in recent decades. Thus, BAC has found more and
more important applications. So far, the applications can be classified into
four major aspects. 1. Selective enrichment of cis-diol-containing small
molecules; 2. Selective enrichment of glycoproteins; 3. Specific detection of
glycoprotein disease biomarkers; 4. Selective enrichment of digested
glycopeptides.
Biotime’s opposition in Boronate
affinity chromatography
Profound product Affinity A1c Analyzer has
been recently introduced by Xiamen Biotime Biotechnology Co., Ltd., which is a
detection system based on reflective colorimetric analysis technology. It is
used with glycosylated hemoglobin reagent (hereinafter referred to as reagent),
by measuring the concentration of the marker corresponding to the reagent,
combined with medicine. The reference value gives quantitative results, which have
the characteristics of accurate detection, fast detection speed, portable use,
and so on. Affinity A1c analyzer is mainly composed of the host.
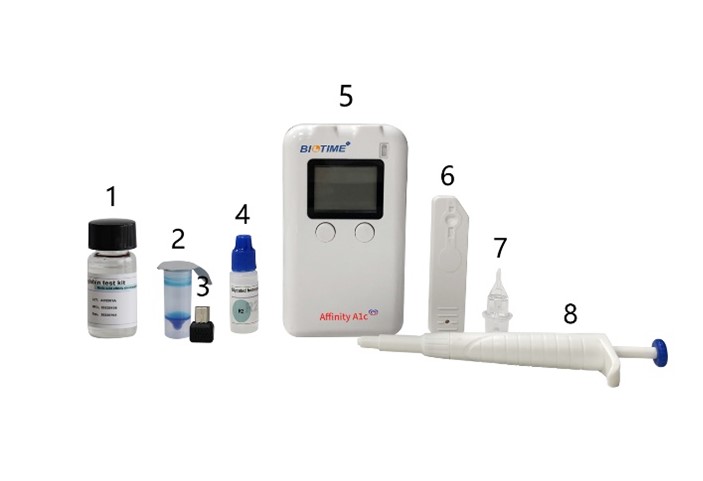
No.
Items
1
R1B
2
R1A
3
Calibration Chip
4
R2
5
Affinity A1c Analyzer
6
Test Cartridge
7
Sampler
8
Transfer Pipette
CONCLUSION AND FUTURE PROSPECTS
This article gives a brief introduction to
BAC. Here we reviewed the basic mechanism of separation and selectivity
employed in BAC. The binding pH, selectivity, and specificity of BAC are
reviewed in detail. A comprehensive understanding of the interaction mechanism
is helpful for better utilization of BAC and boronate affinity materials. As to
future development, we believe the combination of boronate ligands with
structural features will be an important direction. Boronate affinity molecular
imprinting is an example in this direction. We foresee that BAC and boronate affinity
materials will find more and more important applications in the future.
REFERENCES
1. Q. Li, C. Lu, H. Li, Y. Liu, H. Wang, X.
Wang, Z. Liu, ‘Preparation of Organic-Silica Hybrid Boronate Affinity
Monolithic Column for the Specific Capture and Separation of Cis-Diol
Containing Compounds’, J. Chromatogr. A, 1256, 114–120 (2012).
2. R.J. Carvalho, J. Woo, M.R.
Aires-Barros, S.M. Cramer, A.M. Azevedo, ‘Phenyl boronate Chromatography
Selectively Separates Glycoproteins through the Manipulation of Electrostatic,
Charge Transfer, and Cis-diol Interactions’, Biotechnol. J., 9, 1250–1258
(2014). 3. Z. Bie, Y. Chen, H. Li, R. Wu, Z. Liu, ‘Off-line Hyphenation of
Boronate Affinity Monolith-based Extraction with Matrix-assisted Laser
Desorption/ionization Time-of-flight Mass Spectrometry for Efficient Analysis
of Glycoproteins/Glycopeptides’, Anal. Chim. Acta, 834, 1–8 (2014).
4. Q. Zhang, N. Tang, J.W. Brock, H.M.
Mottaz, J.M. Ames, J.W. Baynes, R.D. Smith, T.O. Metz, ‘Enrichment and Analysis
of Nonenzymatically Glycated Peptides: Boronate Affinity Chromatography Coupled
with electron-transfer Dissociation Mass Spectrometry’, J. Proteome Res., 6,
2323–2330 (2007).
5. H. Li, Y. Liu, J. Liu, Z. Liu, ‘A
Wulff-type Boronate for Boronate Affinity Capture of Cis-diol Compounds at
Medium Acidic pH Condition’, Chem. Commun., 47, 8169–8171 (2011).
6. Y. Jiang, Y. Ma, ‘A Fast Capillary
Electrophoresis Method for Separation and Quantification of Modified
Nucleosides in Urinary Samples’, Anal. Chem., 81, 6474–6480 (2009).
7. X.C. Liu, ‘Boronic acids as Ligands for
Affinity Chromatography’, Chin. J. Chromatogr., 24, 73–80 (2006).
8. H. Li, Z. Liu, ‘Recent Advances in
Monolithic Columnbased Boronate-affinity Chromatography’, Trac-Trend. Anal.
Chem., 37, 148–161 (2012).
9. E. Bisse, H.Wieland, ‘Coupling of
m-Amino phenyl boronic Acid to S-Triazine-Activated Sephacryl-Use in the
Affinity-Chromatography of Glycated Hemoglobins’, J. Chromatogr. B, 575,
223–228 (1992).
10. D.C. Klenk, G.T. Hermanson, R.I. Krohn, E.K. Fujimoto, A.K. Mallia, P.K. Smith, J.D. England, H.M. Wiedmeyer, R.R. Little, D.E. Goldstein, ‘Determination of Glycosylated Hemoglobin by Affinity Chromatography: Comparison with Colorimetric and Ion-exchange Methods, and Effects of Common Interferences’, Clin. Chem., 28, 2088–2094 (1982).